Alpha Therapy
with 211At
Why Advancing Targeted Alpha Therapy with 211At
Our project is dedicated to advancing Targeted Alpha Therapy (TAT) with astatine-211, harnessing its distinctive properties to transform cancer treatment.
211At is notable for its chemical properties suitable for labeling targeting vectors ranging from small organic molecules to proteins, and the emission of only one α-particle per decay, providing greater control over off-target effects (Albertsson et al, 2022; Eychenne et al, 2021).
The α-particle emission characteristics of 211At are similar to those of other TAT-candidate radionuclides, exhibiting a mean linear energy transfer and an ionization density close to the diameter of the DNA double helix.
211At decays with a half-life of 7.2 h in two branches: either by α-emission to 207Bi or by electron capture to 211Po, which promptly decays by α-emission to stable 207Pb. This means that each decay yields one α-particle. In addition, this yields polonium X-rays in the range of 70–90 keV, enabling simple quantification, and the energy of the X-rays is in the range where it can be imaged with a γ camera. In contrast to other α-emitters, astatine is a halogen and shares similar chemical properties with iodine, albeit with more metalloid properties. Although significant differences between astatine and iodine have been observed in labeling chemistry, carbon-halogen bond strength, lipophilicity, and the in vivo stability of the carbon-halogen bond, the most common strategy to develop 211At-labeled TAT agents is to build on previous studies with radioiodine (131I, 123I, 124I).
Advantages of 211At over β-emitters are significant.
In terms of properties
211At emits α-particles with high Linear Energy Transfer (LET) (~100 keV/µm), leading to highly localized and effective DNA damage as presented in, particularly suited for targeting cancer cells while sparing surrounding healthy tissue. In contrast, β-emitters have lower LET, resulting in more diffuse energy deposition and potentially greater collateral damage to healthy cells. Moreover, the α-particles emitted by 211At have a very short path length in tissue (50-100 µm), ensuring that radiation is concentrated within a few cell diameters, minimizing off-target effects, whereas β-particles, with their longer path length, can damage surrounding healthy tissues.
In terms of production
211At is produced by irradiating naturally abundant low-cost bismuth targets using a cyclotron, a more straightforward process compared to the need for enriched or scarce materials required for many β-emitters. Additionally, 211At does not require enriched materials, simplifying production logistics.
Safety-wise, due to its short-range α-emission and the absence of long-lived radioactive daughters, 211At presents a lower risk of off-target toxicity compared to β-emitters, which can have more widespread effects due to their longer range in tissues.
Compared to other α-emitters
211At also presents several advantages. Unlike 225Ac or 223Ra, which produce multiple α-emitting daughter isotopes, 211At emits only one α-particle per decay, providing better control over radiation delivery and reducing off-target effects.
211At
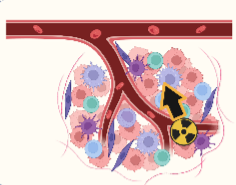
- High LET (50-230 KeV/µm)
- Short Range (20-100 µm)
- Double-strand DNA damage
- One a-particle per decay

Other α-emitters
(e.g., 225AC, 223Ra)
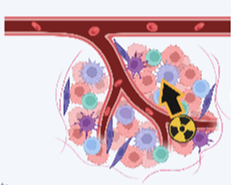
- High LET (50-230 KeV/µm)
- Short Range (20-100 µm)
- Double-strand DNA damage
- Multiple a daughter particles

β-emitters
(e.g., 177Lu, 90Y)
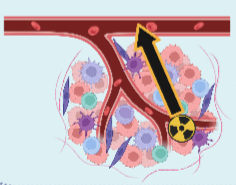
- Low LET (0-2 KeV/µm)
- Low Range (0.05-12 mm)
- Single-strand DNA damage

Figure 1 : Radiation mechanism from different type of radioisotopes (211At, alpha-emitters or beta-emitters).
211At has a more manageable half-life of 7.2 hours, which is longer than some α-emitters like 213Bi (45.6 min) but shorter than others like 225Ac (10 days). This half-life is ideal for clinical applications, allowing sufficient time for complex radiochemistry and patient administration without excessive radiation exposure, while reducing the costs of radiation protection activities.
Scalability is another key advantage. 211At production is relatively scalable using existing cyclotron facilities that can accelerate α-particles. This contrasts with the production of some other α-emitters, which may require more specialized and less accessible facilities. The use of naturally abundant bismuth as a target material further simplifies the production process compared to other α-emitters that require rare or enriched materials.
Safety concerns are lower with 211At due to the absence of long-lived radioactive daughters and the manageable half-life, presenting fewer challenges in radiation protection for both healthcare providers and patients compared to α-emitters like 225Ac, which has more complex decay chains and longer-lasting radiation concerns. Promising early preclinical data has shown that 211At-based therapies are particularly effective for cancers resistant to conventional therapies



